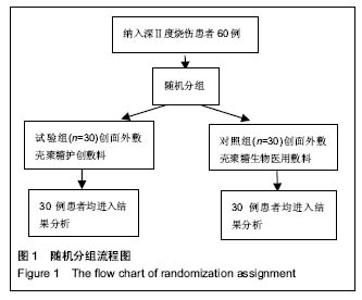
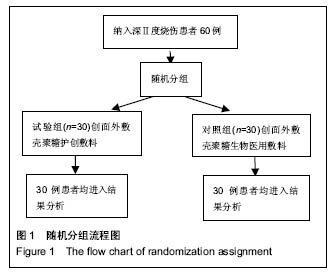
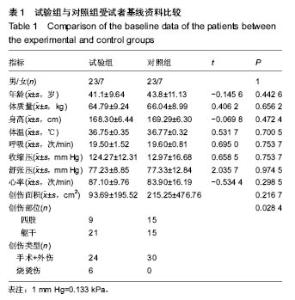
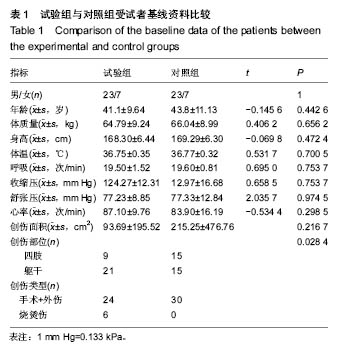
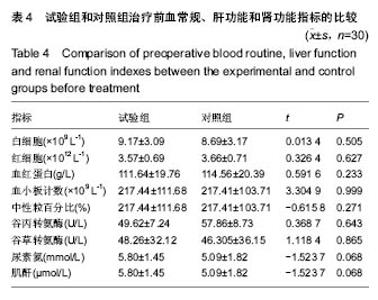
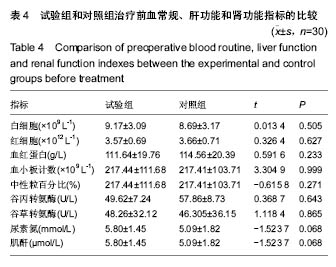
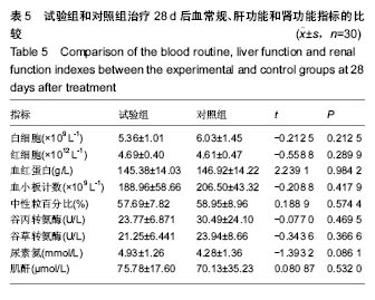
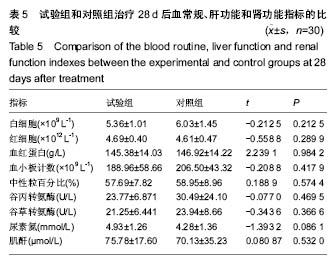
| [1]黎鳌.烧伤治疗学[M].2版.北京:人民卫生出版社,1997:5.[2]袁丹波.壳聚糖抗菌生物医用膜在烧伤创面中的临床应用[J].微生物学免疫学进展,2012,40(1):35-37.[3]朱香利.壳聚糖生物医用材料的应用[J].中外医疗, 2013,32(14): 12-14.[4]杨震.壳聚糖及其衍生物在创伤修复领域的应用进展[J].西部医学,2013,25(2):314-316.[5]蔡良良,吕国忠,陈敬华,等.光交联壳聚糖水凝胶膜治疗深Ⅱ度烧伤创面的临床研究[J].中华损伤与修复杂志(电子版), 2010,5(1): 27-29.[6]黄佩芸.壳聚糖创面修复凝胶在Ⅱ期、Ⅲ期压疮护理中的疗效观察[J].右江医学,2013,41(6):934-935.[7]吴燕珍.壳聚糖衍生物酰氨基葡萄糖在烧伤早期的应用[J].当代护士旬刊,2012,10(10):35-36. [8]李清,杨卫国,陆原.壳聚糖医用膜用于皮肤轻度烧、烫伤治疗的疗效及安全性观察[J].中国医学创新,2012,9(36):38-39.[9]张晓曼.羧甲基壳聚糖湿性敷料对手术切口愈合影响的研究[J].成都医学院学报,2012,7(2):276-278.[10]刘琳娜,李学拥,吴晓春,等.银离子抗菌敷料用于Ⅱ度烧伤创面的疗效和安全性[J].实用药物与临床,2013,16(10):922-924.[11]龚振华,姚建,季建峰,等.银离子敷料联合水凝胶对度烧伤创面愈合的作用[J]中国组织工程研究与临床康复,2009,13(42):8373-8376.[12]赵遵江,薛忠信,章荣涛,等.生物流体膜与湿润烧伤膏对二度烧伤创面疗效的对比[J].安徽医学,2005,26(3):222-223.[13]唐菲,朱毅.六味芦荟膏对感染性烧、烫伤创面的促愈合作用研究[J].中国药房,2006,17(10):733-736.[14]吴晓明,孙艳军,孙奎,等.德莫林联合纳米银医用抗菌敷料治疗老年Ⅱ度烧伤创面的疗效[J].中国老年学杂志,2013,33(6):1291-1292.[15]朱东来,武凤莲,王连英,等.重组人酸性成纤维细胞生长因子与纳米银敷料联合治疗深Ⅱ度烧伤创面的临床观察[J].中国美容医学,2013,22(18):1840-1842.[16]蔡少甫,余昌龙.壳聚糖联合纳米银医用抗菌敷料治疗老年Ⅱ度烧伤创面的疗效观察[J].中国社区医师,2016,32(6):78-79.[17]刘继松,方勇,姚敏,等.重组人粒细胞巨噬细胞集落刺激因子凝胶剂对深Ⅱ度烧伤创面溶痂及愈合影响的临床研究[J].中国修复重建外科杂志,2011,25(9):1059-1062.[18]王配合,杨建民,张轶,等.液超妥与痊愈妥复合敷料治疗Ⅱ度烧伤创面的效果观察[J].临床误诊误治,2011,24(6):17-18.[19]张群,阿不都拉,艾赛提.复方薰衣草油膏治疗Ⅱ度烧伤创面临床对比观察[J].医学理论与实践,2011,24(17):2039-2040.[20]温春泉,赵筱卓,张国安.外用重组人粒细胞巨噬细胞集落刺激因子凝胶治疗深Ⅱ度烧伤创面临床疗效观察[J].中华损伤与修复杂志(电子版),2016,11(3):215-218.[21]孙羽飞,李翔,徐文举.纳米银敷料对深Ⅱ度烧伤创面修复作用的观察[J]. 临床医药文献杂志,2016,3(7):1231-1232[22]王志刚,刘韬,郝朝辉,等.复春散1号与 2 号联合治疗面部深Ⅱ度烧伤的疗效观察[J].内蒙古中医药,2016,35(4):30.[23]王斌,罗旭,唐从容,等.磺胺嘧啶银霜联合重组人粒细胞巨噬细胞刺激因子凝胶治疗深Ⅱ度烧伤创面的疗效评价[J].医药导报, 2015,34(12):1588-1590.[24]张永生.纳米银外用抗菌凝胶联合臭氧治疗深Ⅱ度烧伤创面90例[J].中国药业,2015,24(3):78-79.[25]李林.胰岛素联合重组人酸性成纤维细胞生长因子对深Ⅱ度烧伤创面愈合的效果观察[J].山东医药,2014,54(45):75-76.[26]鲜华,尤婷婷,谭杜勋,等.壳聚糖创面修复膜凝胶门诊治疗Ⅱ度烧伤的临床疗效观察[J].重庆医学,2014,43(36):4959-4961[27]李恒,彭辉.Ⅱ度烧伤创面4种外用药物使用及临床效果比较[J].中国冶金工业医学杂志,2015,32(2):159-160.[28]王勇,王光华.湿润烧伤膏联合重组人表皮生长因子凝胶在Ⅱ度烧伤中的临床应用[J].河南外科学杂志,2015,21(1):91-92.[29]高娅,崔正军,史迅,等.生物流体敷料对Ⅱ度烧伤创面的疗效观察[J].中国美容医学, 2015,24(4):1-3.[30]柴家科,孙永华,夏照帆,等.酸性成纤维细胞生长因子治疗深Ⅱ度烧伤的多中心随机对照临床试验[J].中国药师,2015,18(4):589-591.[31]王强,刘筱雯,吕仁荣,等.生物敷料A对比银离子水胶体油纱修复面部Ⅱ度烧伤[J].中国组织工程研究,2015,19(10):2573-2577.[32]Wang L,Hao C,Zhou B,et al.Application of Heparinized Selective Acellular Sheepskin in Wound-healing Promotion of Deep Second-degree Burns. Wounds. 2016.pii: WNDS20160929-3.[Epub ahead of print][33]Esteban-Vives R,Choi MS,Young MT,et al.Second-degree burns with six etiologies treated with autologous noncultured cell-spray grafting.Burns.2016;42(7):e99-e106.[34]Najmi M,Vahdat Shariatpanahi Z,Tolouei M,et al.Effect of oral olive oil on healing of 10-20% total body surface area burn wounds in hospitalized patients.Burns. 2015;41(3):493-496.[35]Cho YS,Choi YH,Yoon C,et al.Factors affecting the depth of burns occurring in medical institutions.Burns. 2015;41(3): 604-608.[36]Yim H,Yang HT,Cho YS,et al.A clinical trial designed to evaluate the safety and effectiveness of a thermosensitive hydrogel-type cultured epidermal allograft for deep second-degree burns.Burns.2014;40(8):1642-1649.[37]Ouyang J,Chen YC,Luo GX,et al.A randomized and controlled multicenter prospective study of the Chinese medicinal compound Fufang Xuelian Burn Ointment for the treatment of superficial and deep second-degree burn wounds. Cell Biochem Biophys.2014;69(3):467-474. |